In reading we were reading a book about Comic Man and it is about this guy that is making comics ever since he was a kid. It is also about his journey from kid to professional comic writer. Here is the timeline
Monday, 20 September 2021
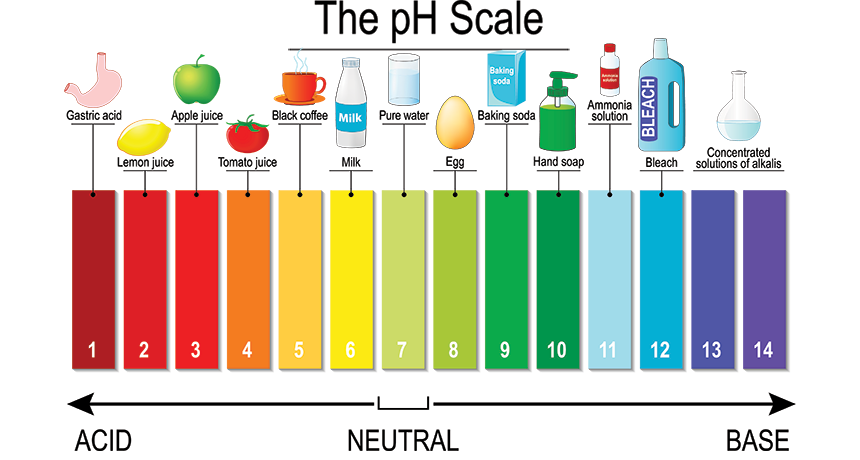
Neutralisation
Acid + Alkaline (Base) = Water & Salt
Equipment:
Universal Indicator
1 M HCl ( Hydrochloric Acid)
1 M NaOH (Sodium Hydroxide)
4 Test tubes
Test tube rack
2 plastic pipettes
Equipment:
Universal Indicator
1 M HCl ( Hydrochloric Acid)
1 M NaOH (Sodium Hydroxide)
4 Test tubes
Test tube rack
2 plastic pipettes
Take a test tube rack with 1 boiling tube at each end of the rack and 4 test tubes.
Half Fill the boiling tube at one end with Sodium Hydroxide.
Half fill the boiling tube at the other end with Hydrochloric acid.
Fill each test tube with 5 drops of Universal indicator.
Using the pipettes you will add drops of the acid or alkaline to the test tubes to make colours. DO NOT MIX UP THE PIPETTES
Thursday, 16 September 2021
Litmus paper
Litmus is a tree lichen fungi and is made into small paper strips or a liquid for testing acids or alkalines.
Blue Litmus paper determines if something is acidic. It turns red in solutions with a pH less than 7.
Red Litmus paper determines if something is alkaline. It turns blue in solutions with a pH greater than 7.
Materials:
Red Litmus paper
Blue Litmus paper
Sodium Hydroxide
Hydrochloric acid
Pipette
4 Test tubes
Rip the litmus paper in half and put half in each test tube.
Add 3 drops of hydrochloric acid to the blue litmus paper in test tube 1.
Add 3 drops of hydrochloric acid to the red litmus paper in test tube 2.
Add 3 drop of Sodium Hydroxide to the blue litmus paper in test tube 3.
Add 3 drops of Sodium Hydroxide to the red litmus paper in test tube 4.
The blue litmus paper with hydrochloric acid went red.
The blue litmus paper with sodium hydroxide didn't change.
The red litmus paper with hydrochloric acid didn't change.
The red litmus paper with sodium hydroxide went blue.
Monday, 13 September 2021
Acid
Aim: To make red cabbage pH indicator for use on different household materials.
Red Cabbage Indicator Experiment:
Materials:
1. Red Cabbage
2. Beaker
3. Bunsen Burner
4. Pipette
Sodium Hydroxide
Hydrochloric acid
Test Tubes
Paper towel
Test tube rack
Water
Steps
Chop up the red cabbage into small pieces. Place in a beaker and cover with a small amount of water.
Bring the solution to the boil using a bunsen burner and then turn off the heat. Let it sit to cool down.
Pour the cabbage water through a paper towel into a beaker. The dark purple liquid in the jar is the pH indicator liquid.
Suck up the indicator into a pipette.
Use the cabbage indicator to test the acidity of the different household liquids by slowly putting a drop at a time into the test tube.